Introductory Experiment
In this experiment you are going to make your own electrochemical cell.
Predict what will be produced in your electrochemical cell.
Write your prediction down before you read on.
Aim |
To make a common electrochemical cell and become familiar with how they work to produce electrical energy
|
Apparatus (Equipment) |
1 x 10 cm steel nail 1 x (10 cm x 1 cm) strip of copper metal 1 x small piece of sandpaper 1 x large lemon or grapefruit 2 x conducting leads with alligator clips 1 x galvanometer or milliammeter (Note: These meters are very delicate so be careful) Note: A 0 to 2 V voltmeter will suffice. Use a galvanometer or alternatively use a multimeter set on the milliamp setting to measure electric current flow and direction. Needle deflection direction indicates current flow with a galvanometer. A negative sign will be present or absent with a multimeter to indicate current direction. If you do use a multimeter you can also check the voltage by changing the meter setting to measure voltage. If you don’t use a multimeter then you will need to have access to both a galvanometer and a voltmeter and switch meters. |
Method |
The nail and the copper strip were polished with some sandpaper and then inserted into small slits in the surface of the lemon, being sure that they did NOT come into contact (as shown below). 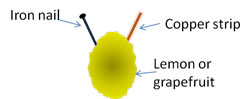
An electrical lead was attached to the nail and another to the copper strip.
The other ends of the leads were then touched briefly to a galvanometer and the result observed.
Take a moment to consider how you can record your results for this investigation.
Predict what will happen if you reverse the connections to the copper strip and nail.
Try this adjustment to your experiment. Record what you observed.
If you have two nails, try using just nails instead of different metals. Record what happens. Try using two strips of copper. Record what happens.
Write a statement about the need to use different metals to produce an electric current in an electrochemical cell.
The wires were then reversed on the galvanometer and again touched to the contacts. The results were recorded. Did they match your prediction?
Change the depth of nail insertion into your piece of fruit and observe whether the size of the electric current produced (as shown on the galvanometer or ammeter) changes. Does it?
Try changing the depth of insertion of the copper strip into the fruit. How can you make the electric current produce a maximum? Can you identify a trend in your results?
The results were then analysed and a conclusion written.
How do you think you should best record your results to make them clear to others? Did you design a table to record your results?
|
Questions
- When comparing this battery (cell) with Volta's, what role does the lemon play? Do you have evidence that supports your statement about the role of the lemon faster the reaction, the more electric current is produced.
- What energy transformations were occurring inside the fruit?
- What effect do you think using other types of metal might have on the results? Make a prediction. If you have another set of metals try these to test your prediction.
- Batteries are commercial electrochemical cells. Identify three things that must be present in a battery to produce an electric current.
- Can you explain why some batteries produce different voltages?
- Can you identify why bigger dry cell batteries that use the same chemicals and metals e.g. D cell batteries (big fat battery) might produce a larger electric current than an AAA cell battery (small thin battery). Explain the difference referring back to the evidence you collected in your investigation.
